HSCT Indication
PREVYMIS® HSCT Indication
PREVYMIS® is indicated for prophylaxis of cytomegalovirus (CMV) reactivation and disease in adult CMV-seropositive recipients (R+) of an allogeneic haematopoietic stem-cell transplant (allo-HSCT).1
In allo-HSCT recipients, the most important risk factors for CMV disease are the serologic status of the donor and recipient. Seropositive recipients (R+) are at a higher risk for transplant-related mortality than a seronegative recipients (R-).2
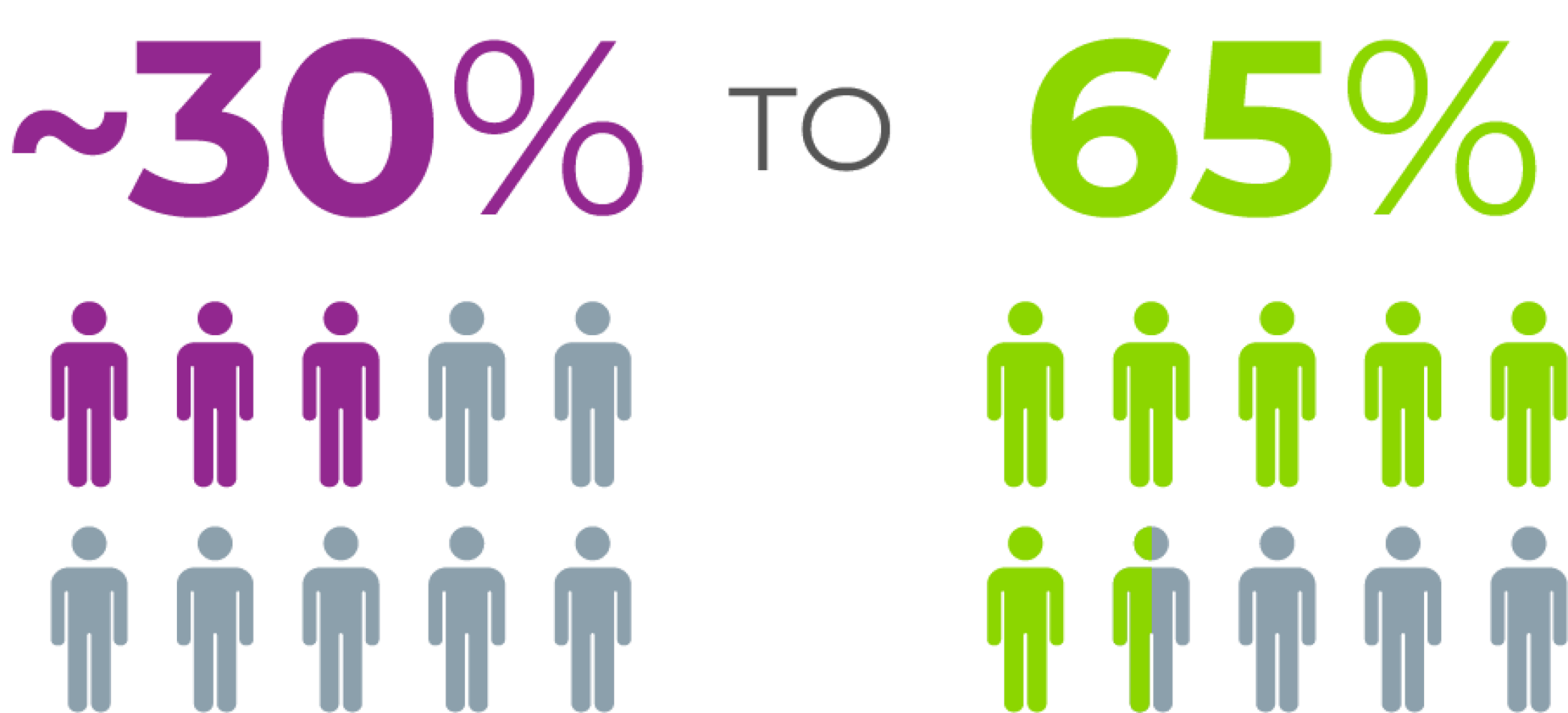
CMV reactivation has been shown to occur in ~30-65% of R+ allogeneic HSCT recipients3,4
Prevalence of CMV infection in R+ allogeneic HSCT recipients4
When to start PREVYMIS®?
In adult patients undergoing allo-HSCT, cytomegalovirus (CMV) reactivation can occur as early as the day of the transplant.2
PREVYMIS® should be started after allo-HSCT. PREVYMIS® may be started on the day of transplant and no later than 28 days post-HSCT. PREVYMIS® may be started before or after engraftment.1
Prophylaxis with PREVYMIS® should continue through 100 days post-HSCT. Prolonged PREVYMIS® prophylaxis beyond 100 days post-HSCT may be of benefit in some patients at high risk for late CMV reactivation.1
References:
- PREVYMIS®. Summary of product characteristics, April 2025.
- Green ML et al.,Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol. 2016 Mar;3(3):e119-27.
- Sousa H et al., Cytomegalovirus infection in patients who underwent allogeneic hematopoietic stem cell transplantation in Portugal: a five-year retrospective review. Biol Blood Marrow Transplant. 2014 Dec;20(12):1958-67.
- Teira P et al., Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood. 2016 May 19;127(20):2427-38.

