Efficacy
PREVYMIS® Efficacy
Study design
CMV prophylaxis was assessed in a randomized, multicenter, double-blind, placebo-controlled, pivotal, phase 3 study of adult CMV seropositive recipients (R+) of allogeneic HSCT. Patients were randomized (2:1) to PREVYMIS® or placebo and stratified by investigational site and risk (high vs low) for CMV reactivation at the time of study entry (n=565).
The protocol suggested thresholds for the initiation of PET during the treatment period were >150 copies/mL for high-risk recipients and >300 copies/mL for low-risk post-transplant, and a threshold of >300 copies/mL for all recipients thereafter period

Adapted from Ljungman P, Schmitt M, Marty FM, et al. A mortality analysis of letermovir prophylaxis for cytomegalovirus(CMV) in CMV-seropositive recipients of allogeneic hematopoietic cell transplantation. Clin Infect Dis. 2020; 70(8):1525- 1533. https://doi.org/10.1093/cid/ciz490 https://creativecommons.org licenses/by/4.0
Standard-of-care CMV preemptive therapy (PET) was initiated if CMV viremia was considered clinically significant
Median time to starting study drug was 9 days after transplantation
Superior efficacy in CMV prophylaxis
The primary end point was the proportion of patients, among patients without detectable CMV DNA at randomization, who had clinically significant CMV infection through week 24 after transplantation. Key prespecified secondary end points were the proportion of patients with clinically significant CMV infection through week 14 and the time to clinically significant CMV infection in the primary efficacy population.
PREVYMIS Demonstrated Superior Efficacy Over Placebo in the Primary Endpoint: Clinically Significant CMV Infection at Week 24 (Non-completer=Failure Approach)
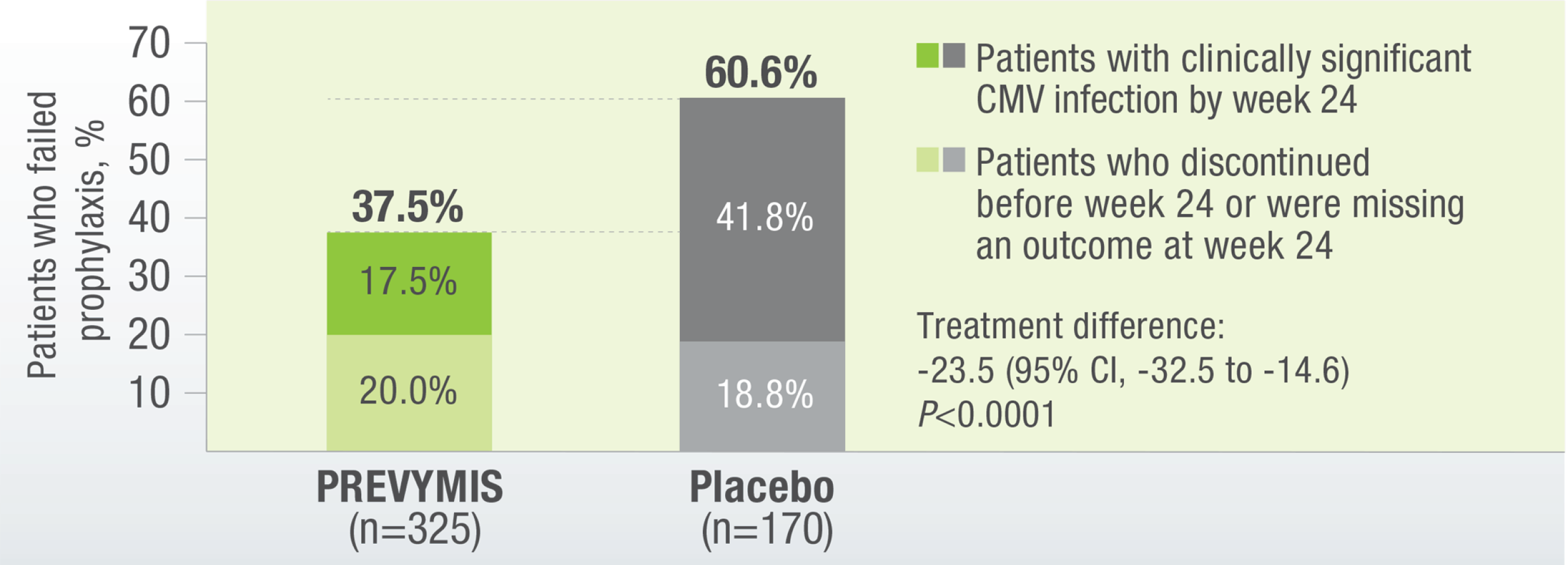
PREVYMIS® demonstrated a significantly lower rate of onset of csCMV infection vs placebo
The secondary endpoint was determined by using the Kaplan-Meier event rate of clinically significant CMV infection among letermovir recipients was 18.9% (95% CI, 14.4 to 23.5), as compared with 44.3% (95% CI, 36.4 to 52.1%) among placebo recipients, by week 24 after transplantation (P<0.001). The following graph shows how effective PREVYMIS® is (vs Placebo) of preventing CMV.1
Time to onset of clinically significant CMV infection through Week 24 post-transplanta

aClinically significant CMV infection was defined as the occurrence of either CMV end-organ disease or initiation of anti-CMV PET based on documented CMV viremia and the clinical condition of the patient.
The following graph outlines the baseline distribution of CMV-positive (CMV R+) transplant recipients, highlighting those with additional risk factors for CMV reactivation.
Recipients at high risk for CMV reactivation2

- Human leukocyte antigen (HLA)- related (sibling) donor with at least 1 mismatch at 1 of the following 3 HLA-gene loci: HLA-A, -B, or -DR
- Haploidentical donor
- Unrelated donor with at least 1 mismatch at 1 of the following 4 HLA-gene loci: HLA-A, -B, -C, or -DRB1
- Use of umbilical cord blood as the stem cell source
- Use of ex vivo T-cell–depleted grafts
- Grade 2 or greater GVHD, requiring systemic corticosteroids
References:
- Marty FM et al., Letermovir Prophylaxis for Cytomegalovirus in Hematopoietic-Cell Transplantation. N Engl J Med. 2017 Dec 21;377(25):2433-2444. doi: 10.1056/NEJMoa1706640. Epub 2017 Dec 6. PMID: 29211658.
- PREVYMIS®. Summary of product characteristics, April 2025.

